Abstract
1. INTRODUCTION
Graft-versus-host disease (GVHD) is the main cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT). First-line treatment is based on the use of high doses of corticosteroids. However, more than 50 % of patients will develop treatment failure with no treatment strategies approved for subsequent lines. Ruxolitinib is an orally administered selective Janus Kinase (JAK) inhibitor approved for the treatment of myelofibrosis and polycythemia vera. It has been previously reported its effectiveness in the treatment of GVHD in pre-clinic models and results in the clinical setting have recently been reported.
2. METHODS
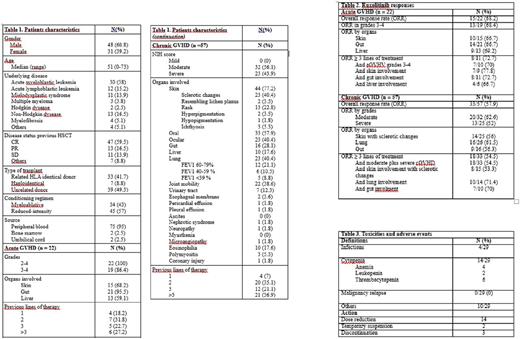
Between October 2015 to July 2017, 79 patients received ruxolitinib under a compassionate use program and were evaluated in this retrospective study using data collected from 13 Spanish centers. The median age was 51 years (range, 0-73). The most frequent underlying diseases were: acute myeloid leukemia (38 %), non-Hodgkin lymphoma (16.5 %) and acute lymphoblastic leukemia (15.2 %). The majority of patients received reduced-intensity conditioning regimens (57 %). Patient baseline characteristics of the entire population are shown in table 1. Patients were scored for their best response at any time after starting ruxolitinib. Treatment responses were considered when patients achieved CR or PR.
3. RESULTS
Twenty-two patients received ruxolitinib for refractory acute GVHD (aGVHD). All patients had grades 2-4 aGVHD and 19 patients (86.5%) had grades 3-4; the median number of previous lines of therapy was 2 (range 1-5). Overall response rate was 68.2% (15/22) which was obtained after a median of 2 weeks of treatment, and 18.2% (4/22) reached CR. We found no differences in treatment responses when analyzed by type of organs involved (table 2). The use of ruxolitinib allowed to taper steroids doses in 16/22 of patients (72%). Cytomegalovirus (CMV) reactivation was observed in 12/22 patients (54.5%). After one year, overall survival was 0.43 (IC: 0.2-0.66).
Fifty-seven patients were evaluated for refractory chronic GVHD (cGVHD). All patients had moderate (32/57, 56.1 %) to severe (25/57, 43.9%) cGVHD. The median number of previous lines of therapy was 3 (range 1-10). Overall response rate was 57.9% (33/57) with 5.3 % (3/57) obtaining CR, with no differences found when analyzed by type of organs involed. Thirty-three patients (57%) could diminished steroids doses a. Cytomegalovirus (CMV) reactivation was observed in 11/57 patients (19.3 %). After one year, overall survival was 0.81 (IC: 0.7 -0.92).
Median dose was 20 mg/day. Overall, 26 patients (32.9 %) discontinued ruxolitinib due to: lack of response (14), cytopenias (3 patients had thrombocytopenia, 3 anemia, 3 had both); infections (1); other causes (2). Only three patients discontinued ruxolitinib due to drug-related toxicities (table 3).
Globally, 18 patients (22.8 %) died: 10/22 patients (45.5%) within the aGVHD and 8/57 patients (14 %) within the cGVHD subgroup. Causes of death were: infections (10), refractory GVHD (6) and other causes (4). Relapse occurred in 1 non ruxolitinib-responsive patient.
4. DISCUSSION
Ruxolinitinb in real life setting has been shown as a effective and safety treatment option for GVHD patients, with ORR of 68.3% and 57.9% for refractory aGVHD and cGVHD respectively in heavily pretreated patients (1-10 previous lines). Ruxolitinib may be an alternative option to consider for steroid-refractory acute and chronic GVHD patients beyond first line treatment.
García Gutiérrez: Incyte: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal